Depuy Lawsuit Lawyer
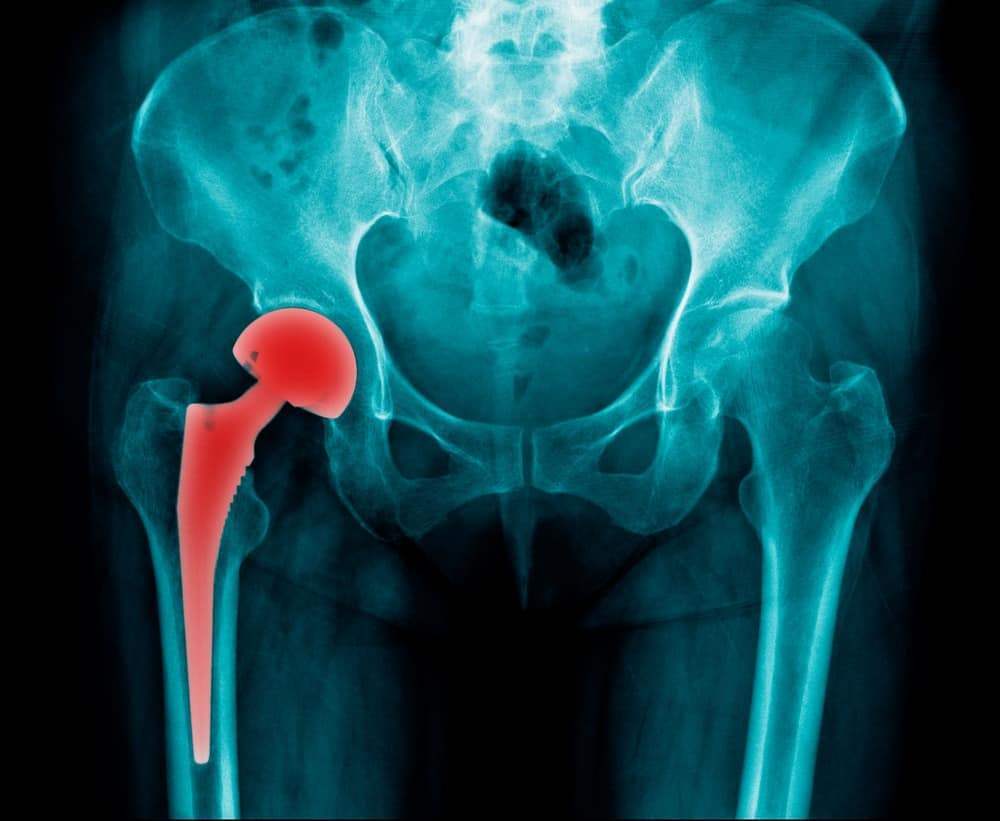
If you have had a hip replacement, you may need a DePuy hip replacement lawyer. On August 26, 2010, DePuy Orthopaedics, Inc. officially recalled two defective hip implant systems, the ASR Hip Resurfacing System and the ASR XL Acetabular System. The recall was ordered by the U.S. Food and Drug Administration (FDA) after receiving a […]

